To fully understand the effects of greenhouse gases, it is necessary to understand the atmosphere. A person could take an entire college course to understand all the interrelationships between atmospheric gases. The basics, however, are not that complicated.
LAYERS OF THE ATMOSPHERE
Because air temperatures may fall or rise with increasing altitude, the atmosphere has well-defined layers. Pay attention to the red line to the right of the chart.
The troposphere begins at the surface of the earth, where the average temperature is 15ºC (59ºF). As altitude is gained, temperature decreases— because the air has less molecules to vibrate — until the -60º C (-76ºF junction with the stratosphere, 4 km up at the poles, 12 at the equator.
In the troposphere, warm air rises, because hotter air is lighter than colder air, transmitting heat rapidly by convection. Water vapor contained in the rising air condenses into clouds and precipitates, giving off heat (the opposite of the heat it took to evaporate it). Water vapor is the most abundant greenhouse gas but we cannot directly change the overall level in the atmosphere as it is controlled by the amount of evaporation from the oceans and precipitation. The atmosphere will hold more only when it is hotter, and the oceans will evaporate more when they are hotter. It is important to understand that water vapor condenses out well below the -60ºC at the top of the troposphere. It is called a condensing gas. Only very extreme turbulence or occasionally a very strong rising air current of hot air at the equator can carry water vapor into the stratosphere (or the once in a millennium eruption of a volcano).
When the stratosphere begins, temperatures rise with increasing altitude. The junction is called the tropopause. This temperature inversion at the bottom of the stratosphere stops transmission of heat by convection (since cold air is heavier than hot air). Non-condensing gases normally cross the tropopause boundary by the very slow process of diffusion (that distributes stable gases throughout the atmosphere). Heat, therefore, continues to space from this altitude almost entirely by radiation. How does the water vapor that does enter the stratosphere get removed? It is removed because at the poles in winter there is no sunlight, and consequently no heat from O2 and ozone. Also the earth underneath is very cold. A down circulation is created from the stratosphere to the troposphere, and the stratospheric air is replaced by rising equatorial air.
The temperature is about -15ºC (-5ºF) at the top of the stratosphere. Why does the stratosphere get hotter with altitude? It is because the sun’s ultraviolet radiation first encounters large numbers of oxygen molecules (O2) at the top of this layer. The UV converts many of these oxygen molecules) to ozone (O3). Both O2 and O3 absorb the UV, and the process creates heat. Since most UV is absorbed at higher altidues, at lower altitudes there is less UV to convert, so temperature decreases.
At still higher altitudes, the temperature again begins to drop as there are now very, very few oxygen and ozone molecules.1 This region of declining temperature is called the mesosphere. The mesosphere, and thermosphere above it, have no effect on climate.
CURRENTS AND WAVES IN THE ATMOSPHERE
You can read a thick book on the weather, as I did, without learning about atmospheric currents other than the Jet Stream. However they have large effects on the weather and also on the transport of methane and water vapor from the troposphere to the stratosphere. This is a highly complex area that is only becoming understood. If you are interested, read up on the quasi-biennial oscillation (QBO), the semi-annual oscillation (SAO), gravity waves, polar vortex waves, sudden stratospheric warming, and mountain waves. Perhaps the most important current is the Brewer-Dobson circulation, which causes air from the equatorial region to rise into the stratosphere, and to be transported to the poles, where it falls back into the troposphere, eventually to be carried towards the equator.2
WHAT ARE GREENHOUSE GASES?
Greenhouse gases are those gases whose molecules absorb outgoing infrared radiation from the earth (and, by some definitions, incoming radiation from the sun).
This occurs because a greenhouse gas molecule may absorb a photon of radiation heading toward space; then either re-emit it in any direction — only occasionally towards space — or convert the energy to motion (heat).
Thus, the photon is thus usually diverted from continuing to space, and if it is not, but it encounters another such molecule, again it will probably be diverted.
The additional molecule is analogous to adding more insulation to a building. (Note that for a building one must double the insulation to decrease heat transfer by a comparable amount and this is also true for the greenhouse gas CO2 that has already widespread in the atmosphere.)
I continually find it astonishing how few of these greenhouse gas molecules (except for plentiful water vapor molecules) there are in the atmosphere. Their prevalence is measured in molecules per million or billion molecules of air.3. It is important that CO2, methane and other greenhouse gases absorb in “windows” where water vapor does not, as shown in the second graphic below.
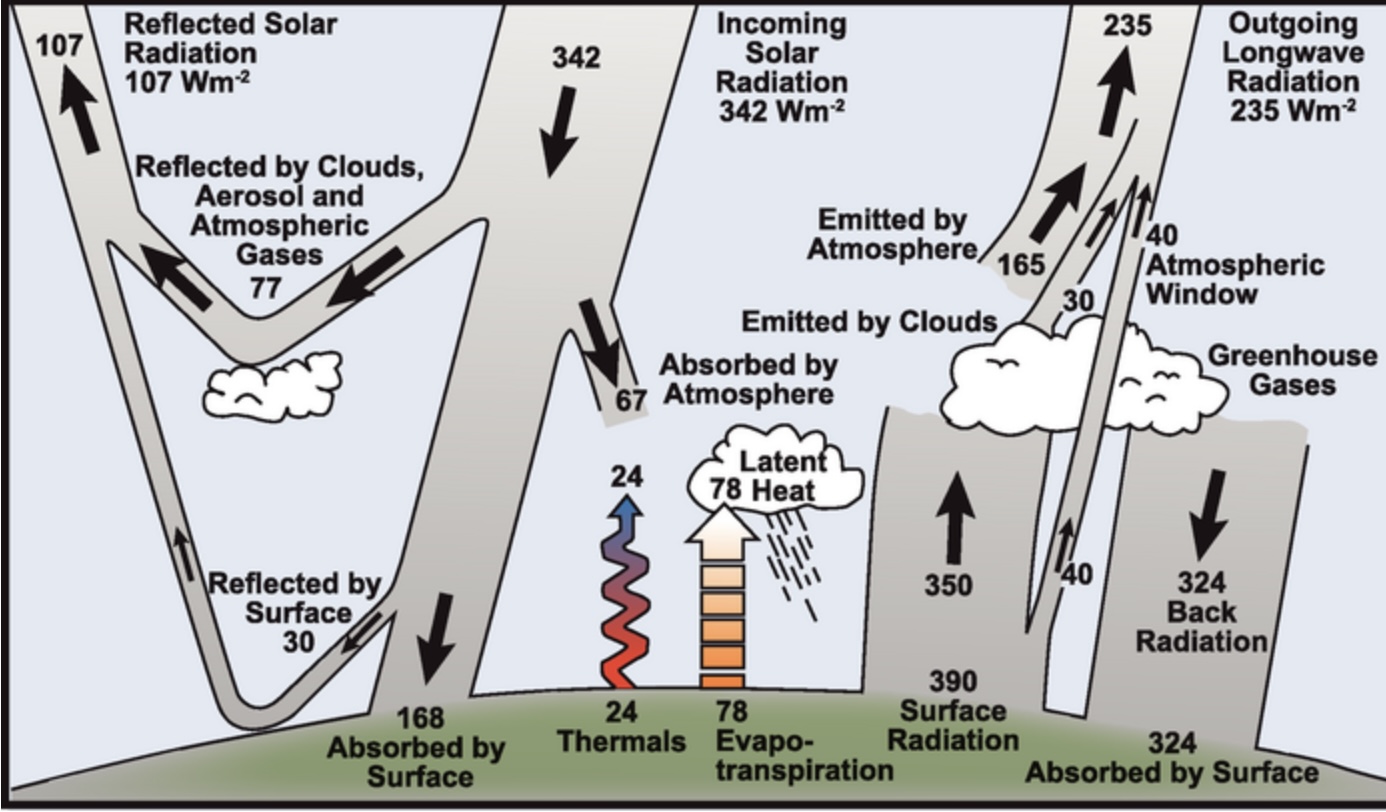
The greenhouse effect is described in many publications as back radiation. This is the radiation that doesn’t escape to space and instead either heats the atmosphere or comes back to the earth’s surface. Although this radiation adds heat to the earth, that heat is less than the radiant heat that the earth is emitting, so you don’t notice it, except in part, perhaps, when the sky is cloudless at night. Its net effect is less cooling of the earth’s surface than would otherwise occur.
The graphic below depicts averages for the entire earth. At any given time some parts of the earth will receive much more solar radiation and/or emit more infrared radiation, for example day versus night, summer versus winter, the tropics versus the poles.
Photons have different energies, which make them have different wavelengths. Each greenhouse gases only absorbs photons in certain narrow bands of the wavelength spectrum. Scientists experiment in laboratories to determine precisely the amount of radiation absorbed by various bands. They use different temperatures and concentrations of gas. Their results are used to make computer models of the atmosphere.
There are many unknowns: At any one location and elevation what is the actual temperature? How much of each greenhouse gas is at that location and do the absorption bands of one gas cancel out the effect of the same bands in another? What processes remove the gas at that location? How long will the gas last? Do additional units of gas have the same effect as previous ones?
Satellites have recently helped answer these questions for most gases. However, only very recently are they able to pinpoint emission sources.
The following graphic roughly indicates how greenhouse gases cover the spectrum of the sun’s and earth’s radiation.
The big blue area around 8-15 microns is known as the atmospheric window because that is where the earth’s radiation is strongest and yet none of today’s most prevalent greenhouse gases block escaping radiation. However, unfortunately, many recently-created chlorocarbon and fluorocarbon gases do — very effectively — and they often have very long lifetimes. That is why these gasses are the most powerful forcing gases, and why some steps have been taken to ban or restrict them.
This conclude my discussion of the science behind greenhouse gases. I look at several greenhouse gases in detail on other pages of this site.
- Source and further discussion here: https://sciencing.com/earths-atmosphere-composition-temperature-19463.html https://sciencing.com/earths-atmosphere-composition-temperature-19463.html ↩︎
- https://acp.copernicus.org/articles/18/4463/2018/ ↩︎
- Data for the most important gases is in a chart on my next page. ↩︎